Background - Allogeneic Hematopoietic Stem Cell transplant (HSCT) is the only potential curative treatment in patients with chronic myelomonocytic leukemia (CMML). Predictors of outcomes of Allo HSCT in CMML vary across studies and include achievement of complete remission, year of transplant, splenomegaly, performance status, prognostic scores and graft source. Inclusion of patients who progressed to AML also confounds outcome comparisons in many previous studies. The factors predictive of outcome with the use of newer GVHD prophylactic regimens including anti thymocyte globulin (ATG) and post-transplant cyclophosphamide (PTCy) are largely unknown. We report our experience of HSCT in CMML in remission and try to identify predictors of survival.
Methods - We retrospectively reviewed all cases of CMML who underwent HSCT at our centre from January 2005 to June 2020. We collected data for demographic characteristics, cytogenetic and molecular characteristics of CMML, prior CMML treatment, latent period before transplantation, prognostic scores, transplant details (donor details, conditioning regimens, GVHD prophylaxis) as well as post-transplant complications (transplant related mortality, occurrence and severity of acute and chronic GVHD, CMV and EBV reactivations). Primary outcome evaluated was overall survival and secondary outcomes were relapse rate and relapse free survival (RFS). Cox-proportional hazards regression model was used to identify predictors of survival.
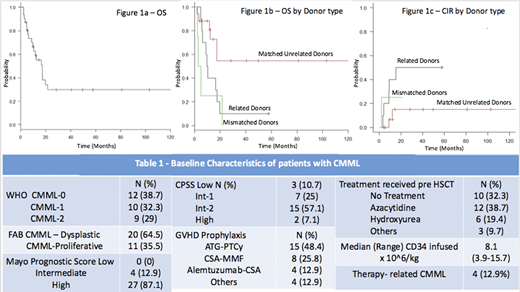
Results - A total of 31 patients underwent allogenic HSCT for CMML during the study period. 58% (n=18) were males. Patients who had progressed to AML were not included in this study. Median age at HSCT was 61 years (range 33-71). Baseline characteristics are summarized in Table 1. Cytogenetic analysis was abnormal in 43.3% (n=13) with high risk cytogenetics (deletion 17p, complex cytogenetics) present in 12.9% (n=4) patients. The median time from diagnosis of CMML to transplantation was 12.4 months. Pre transplant performance status was ECOG Score 0-1 in 73.3% (n=22) patients and Score≥ 2 in 26.7% (n=8) patients. Donors were matched related (MRD), matched unrelated (MUD) and mismatched unrelated MMUD) in 32.3% (n=10), 54.8% (n=17), 12.9% (n=4) transplants respectively. Donor age was significantly lower in MUD when compared to related donors (Median donor age 27 vs 54.5 years; p=0.01). Conditioning regimens used were myeloablative in 25.8% (n=8) and reduced intensity in 74.2% (n=23) patients. The most common GVHD prophylactic regimens used were ATG-PTCy based in 45.2% (n=14) patients. Transplant related mortality (TRM) was 9.67% (n=3). Acute and chronic GVHD occurred in 38.7% (n=12) and 48.4% (n=15%) respectively. After a median follow-up of 12.1 months, 25.8% (n=8) patients relapsed. Estimated 1-year Relapse free survival (RFS) and overall survival (OS) were 52.4% and 59% respectively (Figure 1A). Having a 10/10 MUD was the only significant predictor for improved OS (median OS in MUD vs MRD vs MMUD = 140 vs 10 vs 4 months; p=0.014) and RFS (median RFS in MUD vs MRD vs MMUD = 140 vs 9 vs 2.3 months; p= 0.01) after Cox Regression Analysis (Figure 1B). Presence of a MUD also predicted for a lower cumulative incidence of relapse when compared to MRD or MMUD at 1-year post HSCT (6.8% vs 40% vs 25% respectively; p=0.049) (Figure 1C). GVHD prophylactic regimens containing Alemtuzumab or ATG-PTCy showed a trend towards improved RFS and OS when compared to other GVHD prophylactic regimens (median RFS 17.4 vs 10 months; p=0.19). Cytogenetic risk stratification, donor age, and CMML prognostic scores (Mayo/MDACC/CPSS) were not predictive of survival.
Conclusions - Allogeneic hematopoietic stem cell transplantation remains the only curative modality for patients with CMML. Use of matched unrelated donors may improve outcomes after allogeneic HSCT in patients with CMML, primarily by reducing the cumulative incidence of relapse. Younger donor age and increased use of in vivo T cell depletion in unrelated donor transplants may have contributed to the improvement in outcome.
Lipton:Ariad: Consultancy, Research Funding; Bristol-Myers Squibb: Honoraria; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Mattsson:Gilead: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; ITB: Honoraria; Mallinkrodt: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal